Mitre Flats –
tramping with science
A hiking trip into Mitre Flats, Tararua Range, to use some simple experiments to explain everyday phenomena in the hills
Wind chill (W) = 13.12 + (0.6215t) – (11.37k0.16) + (0.3965tk0.16)
Have you ever wondered why a rainbow disappears when you view it through polarised sunglasses? Or why the rivers flowing out of the Tararua towards the east all make a kink 7 km to the south. Or how to find your altitude by boiling a billy of water. Or why you need to pyrolise wood before a campfire will burn; why you feel colder when there is a wind blowing; how much heat is lost through seams in your clothing and sleeping bag; how you can tell south by the stars; do survival blankets retain body heat; and what little critters may be lurking outside your tent during the night?
Well, Kate did—and together with Illona, Karen, Uta, Kevin, Pete G, Tony G, and Juan—set out on a trip to Mitre Flats to try and find the answers. We were carrying normal tramping gear, plus sunglasses, geological maps, thermometers, a thermal imaging camera, anemometer, and tracking tunnels. And we were all in the mood to think about, and discuss, what was going on around us and why. We made some observations, did a few experiments, asked each other lots of questions, and came back a bit more enlightened after a very different sort of fun weekend in the hills. And we even got off to a great start when a rainbow appeared just as we were packing to leave the Pines.
Experiment 1.
Aim: To understand why a rainbow disappears when viewed through polarised sunglasses.
Apparatus: Tramper, sunglasses, rainbow.
Method: View rainbow normally, then through sunglasses, then again with head tilted 90 degrees.
Result and explanation. Polarised sunglasses are polarised vertically (to reduce reflections from wet horizontal surfaces such as water). The light from a rainbow is also polarised (by reflection and refraction in raindrops) but is essentially polarised in the direction of the arc, i.e. horizontally at the apex. So the top part of a rainbow viewed through polarised glasses will not be visible—except if you tilt you head 90 degrees and then your glasses are effectively polarised horizontally and hey presto the rainbow reappears.
Experiment 2.
Aim: To determine why the track to Mitre Flats alongside the Waingawa River heads north whereas further upstream, and lower down, the river heads more easterly towards the plains.
Apparatus: Tramper, Waingawa River, map.
Method: Check the track direction with respect to the direction of the major fault lines traversing the Tararuas, especially the Wellington Fault.
Result and explanation. The Wellington Fault comes ashore on Wellington’s south coast, carries on up Long Gully and through Karori, under Parliament Buildings and the ferry terminal, along the western side of the Hutt Valley, past Kaitoke—where it takes a 2 km side-step to the east—up the Tauherenikau Valley, over Cone Saddle, through Totara Flats, continues through Pig Flat, the Atiwhakatu, and then crosses into the Waingawa Valley at the low point in Pinnacle Ridge just where the climb to Baldy starts. It continues to Cow Saddle before finally leaving the Tararuas at Putara and becoming the Mohaka Fault after it crosses the Manawatu River. So the Barra Track follows the river where it has eroded itself a valley along the line of the fault where the rock is crushed and weakened by fault movements.
Geologists are able to decipher the clues that are left by fault movements and from these estimate the rate that they are moving and the date the fault was last active. The date for the most recent movement of the segment of the Wellington Fault between Wellington and Kaitoke is generally well known and accepted as approximately 400 years ago, and for the segment from Putara northwards as approximately 240 years ago. But until recently there was no data for the Tararua segment between Kaitoke and Putara. Now, however, after a number of boreholes were sunk at Totara Flats there is a clearer idea of how the flats came to be, and the most recent movement of this segment is now thought to be approximately 1,150 years ago.
If you get a Tararua map and cut along the line of the Wellington Fault and then move the western piece about 7 km south you will find the main rivers such as the Ruamahanga, Waingawa, Waiohine, and Tauherenikau join up again and run directly eastwards without a southward kink. And this is because over the life of the Wellington Fault the western side, in the Tararuas at least, has moved about 7 km north relative to the eastern side—and the rivers have been forced to flow that far south along the line of the fault before being able to once again flow eastwards out towards the Wairarapa Plains.
For more on the faults of the Tararua Range go here.
Experiment 3.
Aim: To estimate altitude by boiling water.
Apparatus: Tramper, hill, billy and cooker, thermometer.
Method: Measure the temperature of boiling water in Mitre Flats Hut. Walk part way up the track to Mitre and then repeat.
Result and explanation. The boiling temperature of water decreases with increasing altitude. At the top of Mt Everest the boiling temperature is only about 71°C. One of the early climbers of Taranaki/Mt Egmont estimated the height of the mountain by carrying up firewood, boiling a billy, and measuring the water temperature (accurate aneroids did not exist at the time), and came up with a height reasonably close to today’s surveyed height.
The expression for calculating the temperature of boiling water at any pressure is:
TB = [(1/TO) – (RU/lU) ln(PB/eO)]-1
where TB is the boiling temperature at PB (Kelvin), lU is the latent heat of vapourisation, RU is a constant (for vapour), TO is the triple point temperature, and eO is the triple point saturated vapour pressure.
This is not a formula you would likely carry around in your head—and anyway lU varies with temperature, and additionally the pressure at any altitude is not constant but varies with atmospheric conditions. But there are tables available that give the change in boiling point with the change in height, so assuming the air pressure doesn’t change as you ascend (or descend), and you know the boiling temperature of water at the point you started, then you can estimate your new altitude by the new boiling temperature of the water. Simple.
But what is not so simple is understanding the weird properties of water—a substance we are so familiar with we tend to take it for granted. One essentially unique feature is that solid water (ice) is less dense than liquid water—ice bergs happily float in water. Almost no other substances have this property. Water exists only because of the ability to form intermolecular hydrogen bonds—without this it would be a gas at room temperature. Equally odd is that water can exist as a solid, liquid or a gas within the temperatures we as humans exist on planet Earth.
Another weird and important property is its high specific heat. This means that large bodies of water such as oceans and lakes can moderate the surrounding climate by absorbing heat in summer and releasing it in winter with very little change in the temperature of the water.
As water freezes it increases in volume—another unusual property, and one that leads to rapid weathering of our mountains as water seeps into cracks in the rocks, freezes and expands, and fractures the rock.
And for snowcrafters in the club—when water freezes to form ice it can be one of about 18 different phases (atomic packing geometries). So remember that the ice you are climbing is not just any old ice.
Anyway, we measured the boiling temperature of the water in our billy at 99.9°C at Mitre Flats Hut, and then 99.6°C after climbing someway up the track (actually not very far as there was a lack of enthusiasm for going higher— perhaps it was the passing rain showers).
Although we knew the expression for calculating the change in temperature with altitude we carried a copy of the printed simple tables to make it easy. From the tables and with our measured difference in boiling temperature we figured we had climbed about 500 m. From the map and the GPS we were actually 440 m above the hut. So, given that our thermometer was accurate only to 0.1°C and we had not climbed very high we were happy with the result.
P.S. Given that boiling is actually an energetic form of evaporation that occurs when the vapour pressure of the water is equal to the atmospheric pressure, it is interesting to speculate on what would happen if water were emptied into outer space where the pressure is effectively zero, and the temperature can be considered as near enough to zero degrees Kelvin? Well it sort of boils and freezes at the same time—actually it boils and then instantly freezes—to produce ice crystals.
Experiment 4.
Aim: To understand the three requirements for burning—fuel, oxygen, and heat, and especially the need for heat. Apparatus: Cold tramper, firewood, fireplace, lighter or matches.
Method: Start a fire with small kindling then gradually increase size of wood. Continue until tramper is toasty.
Result and explanation. We started a very small fire using thin dry sticks over the standing flame from a small piece of burning bicycle tube. As the fire slowly developed larger sticks could be added until after about 20 minutes quite large logs were used and the fire began to give off significant heat.
Most solids, including wood, do not burn but first require chemical decomposition (pyrolysis) to yield gaseous products of sufficiently low molecular weight that will enter a flame, oxidise, and release heat.
Wood is a complex mixture of natural polymers of high molecular weight, the most important being cellulose and lignin. Plus wood contains moisture that needs to be evaporated at 100°C before the wood can be heated further. To begin to thermally decompose the wood needs to be heated to about 450°C with the simplified chemical equation:
6C10H15O7 + heat → C50H10O + 10CH2O. These products then react with oxygen in the air:
CH2O + O2 → H2O + CO2 + CO + C + heat. Some of this heat generated is reflected back onto the fire which then causes a positive feedback and the fire develops.
So if you are having trouble lighting a fire just think that beyond fuel and oxygen you need sufficient heat to initially evaporate any water in the wood, then heat the wood to about 450°C before any combustible volatiles are produced. Then have as much of the heat as possible reflected and radiated back into the fire and not outwards into space so as to increase the rate of pyrolysis. This is why a well-constructed fireplace of stones around three sides to return significant amounts of heat radiated from the flames back into the fire will make for a hotter, more efficient fire.
This brought us to the gnarly question of burning plastic waste. Most on the trip were against it—but one person offered the idea that given that most single-use plastic shopping bags are manufactured from polypropylene which is derived from natural gas or petroleum and has the simple chemical formula of (C3H6)n and when burnt gives mainly carbon dioxide and water, it is therefore actually no more harmful than burning wood. Plastic containers are mostly PET (polyethylene terephthalate) which has the formula (C10H8O4)n. When burnt it can produce the usual H2O + CO2 + CO + C + heat, but additives may give aldehyde, ethylene, benzene, and biphenyl. Although some of these are rather nasty they are produced in quite low volumes especially if the fire is very hot.
However, other plastics such as PVC, polyurethane and even polystyrene, that may contain nitrogen, do release significant amounts of particularly nasty products including cyanide and should not be discarded in a campfire but carried out and disposed in a landfill. Or better still not used at all.
But—discarded single-use supermarket shopping bags are perhaps best burnt in a blazing hot campfire to perhaps avoid them escaping into the environment where they are often a lethal hazard to wildlife. Probably yes? No?

Smoke: Minute carbon particles (soot) plus combustion gases
Flames: The exothermic reactive part of the fire—gases at approximately 950°C emit heat plus visible light
Fuel: Wood heated to above 450°C yields combustible volatiles
Fireplace: Built with the open side facing the wind to increase supply of oxygen. The closed sides re-radiate heat into the fire to increase the rate of pyrolysis.
Experiment 5.
Aim: To understand wind chill and measure the heat lost through the clothing.
Apparatus: Tramper, cold weather clothing, infrared camera.
Method: Check the expression used by weather forecasters to determine wind-chill and understand what it means and photograph tramper in various states of dress with thermal imaging camera to determine heat leakage areas.
Result and explanation. The MetService uses the expression given in the above subtitle to this post to determine wind chill (a term they also call the ‘feels like’ temperature), where W is the wind chill, T is the measured still air temperature, and K is the wind speed. There are a number of simplifications and assumptions in this calculation (such as your skin is dry) but nevertheless it is a useful measure for trampers. For example, if the wind-chill at Powell Hut was given as 4°C, it would mean the temperature you would feel outside in the wind would be equivalent to that if you shut yourself in your fridge. However, the temperature you would measure inside the hut without the wind may be something like 10°C. Knowing likely wind-chill temperatures for alpine trips is useful, for example, with a wind-chill of about -20°C bare dry skin will become frostbitten in less than 30 minutes, and this can occur with a wind of 50 km/hr and temperature of -12°C which are not particularly unusual conditions in the New Zealand mountains.
Using the thermal imaging camera we then took photos of trampers with various clothing. For a typical tramper in shorts with a single layer top and no hat the heat profile is shown in the photo below for the 3rd and 5th person from the left. Internal body temperature is about 37°C while skin temperature is significantly less. The infra-red photo was taken at 8:27pm (it was cold and pitch dark) and the ground temperature is shown as -1°C.
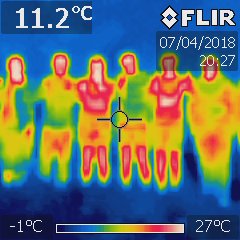
The two cold trampers have only a single layer light top and shorts and were clearly losing a lot of warmth (the red colour indicates that the outside of their clothing was warm and therefore not insulating them). The middle tramper of that group had two top layers. The others were wearing jackets and longs. No-one had a hat. Everyone was losing heat from their face and head while those with bare legs were clearly losing a lot of extra heat. But nearly everyone was losing heat through their clothes from around their groin and armpits (the areas that are naturally warmest) and additionally the right side person was losing heat through the seams in the arms of their jacket.
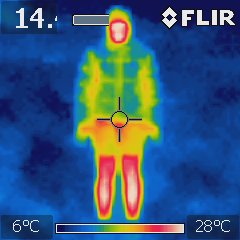
In the photo above the tramper was wearing a thick padded jacket with a hood but still had shorts. They were losing significantly less heat from their head but the seams in the jacket are not well thermally sealed as there was heat leaking along all the seam lines. Again, they were losing heat from uncovered face and bare legs as expected but were significantly better off than those in the top photo.
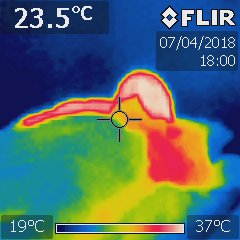
The photo above is the top third of a person lying on their back in their sleeping bag looking towards their head. Clearly there was a shortage of down in their bag around their shoulders with significant heat loss and the bag needs to have the down shaken to move it back to avoid this. The bag was also leaking heat along the line of the zip (right side in the photo) which was a design flaw. The photo also shows the importance of pulling the hood draw cord fully closed around your face in cold conditions—this person was losing significant heat from their very warm face (about 34°C)which needs to be covered if you want to stay really toasty warm.
We took lots of additional infra-red photos of various combinations of clothing and sleeping bags and the results were fascinating, but all tended to show that it was important to pay attention to the small details if you want to stay warm in cold conditions.
Experiment 6.
Aim: To record and identify the track of some of the little critters that come sneaking around your tent late at night while you are sleeping.
Apparatus: Trapping tunnels with ink pads, bait.
Method: Set up a number of baited tunnels and check the white bases for inked foot prints in the morning.
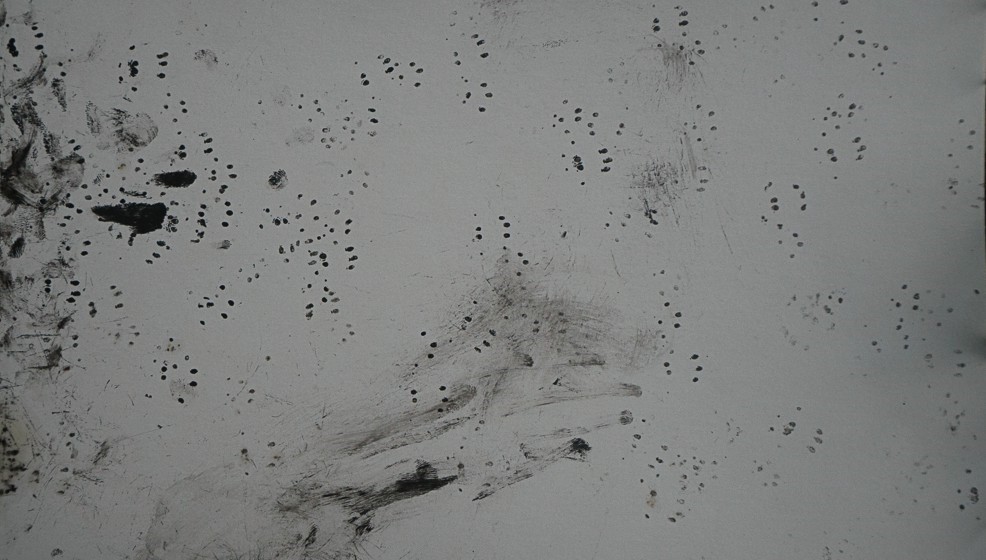
Result and explanation. All the tunnels had multiple foot prints and the baits mostly eaten. Illona could identify many of the prints. They included the expected mice, rats and some beetle tracks. One tunnel had been pulled apart to allow something to get at the bait—there was some discussion as to what this may have been.
The photo shows the tracks from one of the tunnels with the ink foot prints of mice and rats and additionally some tracks made by beetles.